First-principles investigation of the electronic properties of the Bi2O4(101)/BiVO4(010) heterojunction towards more efficient solar water splitting†
Abstract
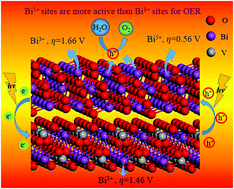
First-principles calculations based on density functional theory were carried out to explore the geometric structure, light absorption, charge separation, over-potential and stability of Bi2O4 (101)/BiVO4 (010) heterojunction. The results show that the formed heterojunction can improve visible light utilization and promote transfer of photo-generated holes from BiVO4 to Bi2O4. Furthermore, the Bi5+ site in the Bi2O4(101) surface is energetically more favorable as the photoanode for the oxygen evolution reaction (OER) than the Bi3+ sites in Bi2O4(101) and BiVO4(010). At the same time, it is also found that the Bi5+ in Bi2O4(101) are more stable than the Bi3+ due to the lower surface energy and stronger bond energy with neighbors. Therefore, forming the Bi2O4/BiVO4 heterojunction can effectively improve the activity and stability of BiVO4 for water splitting reactions.


 Please wait while we load your content...
Please wait while we load your content...