Unravelling the configuration of transient ortho-quinone methides by combining microfluidics with gas phase vibrational spectroscopy†
Abstract
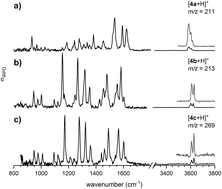
The alkylidene double bond configuration of transient ortho-quinone methides (o-QMs) is studied by cryogenic ion trap vibrational spectroscopy. To this end, o-QMs are formed from ortho-hydroxy benzhydryl alcohols in a Brønsted acid mediated dehydration reaction on a microfluidic chip reactor and the E/Z isomer ratio is determined by comparing the measured gas phase mid-infrared fingerprints with the predicted harmonic spectra from density functional theory calculations. Control over the stereochemistry is achieved by exploiting steric repulsion interactions between the substituents adjacent to the formal double bond of the o-QMs. Attempts to manipulate the ratio of the E- and Z-isomers by varying the reaction conditions were unsuccessful. This observation suggests a low isomerization barrier and hence shorter equilibration times with respect to the on-chip residence time. The fluxional character of the formal double bond is confirmed in 13C-labelling experiments, which reveal a substantially red-shifted CC stretching frequency characteristic for extended, conjugated π-systems.


 Please wait while we load your content...
Please wait while we load your content...