Influence of molecular design on radical spin multiplicity: characterisation of BODIPY dyad and triad radical anions†
Abstract
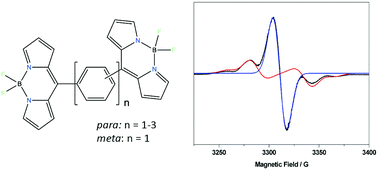
A strategy to create organic molecules with high degrees of radical spin multiplicity is reported in which molecular design is correlated with the behaviour of radical anions in a series of BODIPY dyads. Upon reduction of each BODIPY moiety radical anions are formed which are shown to have different spin multiplicities by electron paramagnetic resonance (EPR) spectroscopy and distinct profiles in their cyclic voltammograms and UV-visible spectra. The relationship between structure and multiplicity is demonstrated showing that the balance between singlet, biradical or triplet states in the dyads depends on relative orientation and connectivity of the BODIPY groups. The strategy is applied to the synthesis of a BODIPY triad which adopts an unusual quartet state upon reduction to its radical trianion.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...