An IRMPD spectroscopic and computational study of protonated guanine-containing mismatched base pairs in the gas phase†
Abstract
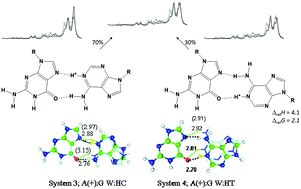
Infrared multiple photon dissociation (IRMPD) spectroscopy has been used to probe the structures of the three protonated base-pair mismatches containing 9-ethylguanine (9eG) in the gas phase. Computational chemistry has been used to determine the relative energies and compute the infrared spectra of these complexes. By comparing the IRMPD spectra with the computed spectra, in all cases, it was possible to deduce that what was observed experimentally were the lowest energy computed structures. The protonated complex between 9eG and 1-methylthymine (1mT) is protonated at N7 of 9eG—the most basic site of all three bases in this study—and bound in a Hoogsteen type structure with two hydrogen bonds. The experimental IRMPD spectrum for the protonated complex between 9eG and 9-methyladenine (9mA) is described as arising from a combination of the two lowest energy structures, both which are protonated at N1 of adenine and each containing two hydrogen bonds with 9eG being the acceptor of both. The protonated dimer of 9eG is protonated at N7 with an N7–H+–N7 ionic hydrogen bond. It also contains an interaction between a C–H of protonated guanine and the O6 carbonyl of neutral guanine which is manifested in a slight red shift of that carbonyl stretch. The protonated 9eG/9mA structures have been previously identified by X-ray crystallography in RNA and are contained within the protein database.


 Please wait while we load your content...
Please wait while we load your content...