Trimethylamine N-oxide (TMAO) resists the compression of water structure by magnesium perchlorate: terrestrial kosmotrope vs. Martian chaotrope†
Abstract
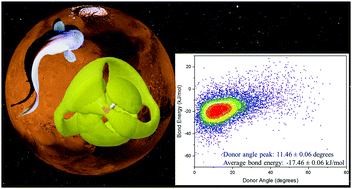
The presence of magnesium perchlorate (Mg(ClO4)2) as the dominant ionic compound in the Martian regolith and the recent discovery of a subsurface lake on Mars suggests that beneath the Martian surface may lie an aqueous environment suitable for life, rich in chaotropic ions. Closer to Earth, terrestrial organisms use osmolytes, such as trimethylamine N-oxide (TMAO), to overcome the biologically damaging effects of pressure. While previous studies have revealed that Mg(ClO4)2 acts to modify water structure as if it has been pressurized, little is known about the competing effects of chaotropes and kosmotropes. Here we ask whether TMAO can help to preserve the hydrogen bond network of water against the pressurising effect of Mg(ClO4)2? We address this question using neutron scattering, computational modelling using Empirical Potential Structure Refinement (EPSR) analysis, and a new approach to quantifying hydrogen bond conformations and energies. We find that the addition of 1.0 M TMAO to 0.2 M Mg(ClO4)2 or to 2.7 M Mg(ClO4)2 is capable of partially restoring the hydrogen bond network of water, and the fraction of water molecules in energetically unfavourable conformations. This suggests that terrestrial protecting osmolytes could provide a protective mechanism to the extremes found in Martian environments for biological systems.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...