Lattice and thermodynamic characteristics of N-stearoyl-allo-threonine monolayers
Abstract
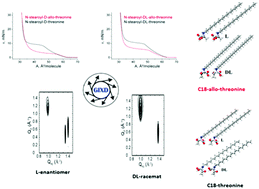
The effect of the second chiral center of diastereomeric N-alkanoyl-allo-threonine on the main monolayer characteristics has been investigated. The characteristic features of the enantiomeric and racemic forms of N-stearoyl-allo-threonine monolayers are studied on a thermodynamic basis and molecular scale. The π–A curves of the enantiomeric and racemic allo-forms show similar features to those of N-stearoyl-threonine. The compression curves are always located above the corresponding decompression curves and the decompression curves can be used as equilibrium isotherms for both the enantiomeric and racemic N-stearoyl-allo-threonine. The absolute T0-values (disappearance of the LE/LC-transition) are 4–5 K larger compared with the corresponding N-stearoyl-threonines, but the ΔT0 between the enantiomeric (D) and the racemic (DL) forms is only slightly larger than that of N-stearoyl-threonine. The difference in the critical temperatures Tc, above which the monolayer cannot be compressed into the condensed state, between the enantiomeric and the racemic forms, is quite small (ΔTc = 0.8 K) and is smaller compared to that of the corresponding threonines (ΔTc = 1.8 K). This is consistent with the dominance of the van der Waals interactions between the alkyl chains reducing the influence of chirality on the thermodynamic parameters. GIXD studies of N-stearoyl-allo-threonine monolayers provide information about the lattice structure of condensed monolayer phases on the Angstrom scale and stipulate the homochiral or heterochiral preference in the condensed phases. Comparable to N-stearoyl-threonine, the enantiomers exhibit an oblique lattice structure, whereas the racemates form a NNN tilted orthorhombic structure demonstrating the dominance of heterochiral interactions in the racemates independent of the diasteomeric structure change of the polar head group. The A0 values are characteristic for rotator phases. The smaller A0 value obtained for the racemic monolayers indicates their tighter packing caused by heterochiral interactions. The program Hardpack was used to predict the geometric parameters of possible 2-dimensional packings. For comparison with the experimental GIXD data, the two-dimensional lattice parameters and characteristic features of the enantiomeric and racemic diastereomeric stearoyl-threonine monolayers were calculated and are in reasonable agreement with the experimental GIXD data.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...