New mechanistic pathways for the formation of organosulfates catalyzed by ammonia and carbinolamine formation catalyzed by sulfuric acid in the atmosphere†
Abstract
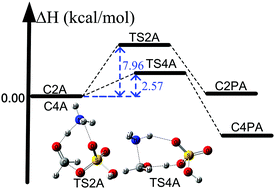
In the secondary organic aerosol formation, exploring the formation of nucleation precursors is of paramount importance for understanding the secondary organic aerosol formation. Here, we report new mechanistic pathways for the formation of organosulfates and carbinolamine in the atmospheric gas phase by utilizing a high-level W2X//QCISD/cc-pV(T+d)Z method close to CCSD(T)/CBS accuracy, dual level kinetics strategy by combining multistructural variational the transition state theory, containing small-curvature tunneling at the M08-SO/maug-cc-pVTZ level with the conventional transition state theory at the W2X//QCISD/cc-pV(T+d)Z level, and torsional anharmonicity. However, a previously suggested mechanism indicated that these are only formed in the heterogeneous atmospheric chemical processes. Furthermore, we find that ammonia only exerts a catalytic role in the HCHO + H2SO4 reaction responsible for the formation of organosulfates under some atmospheric conditions, whereas sulfuric acid can significantly promote the HCHO + NH3 reactions, resulting in the formation of carbinolamine in the atmosphere. These calculated results also show that the ammonia-catalyzed reaction of formaldehyde with sulfuric acid can play an important role in the loss of formaldehyde and the sulfuric acid-catalyzed reaction of formaldehyde with ammonia can also contribute towards the loss of ammonia under some atmospheric conditions. In theory, detailed computational strategies have been designed to obtain quantitative rate constants for the reactions investigated here. A remarkable decrease in the rate constant of the reaction between HCHO and H2SO4⋯NH3 is also observed due to recrossing effects. In addition, the calculated results show that M08-SO functional can provide reliable results for describing the reactions studied here with unsigned error bars of less than 1 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...