Length-scale dependence of protein hydration-shell density†
Abstract
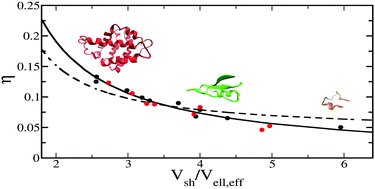
Here we present a computational approach based on molecular dynamics (MD) simulation to study the dependence of the protein hydration-shell density on the size of the protein molecule. The hydration-shell density of eighteen different proteins, differing in size, shape and function (eight of them are antifreeze proteins), is calculated. The results obtained show that an increase in the hydration-shell density, relative to that of the bulk, is observed (in the range of 4–14%) for all studied proteins and that this increment strongly correlates with the protein size. In particular, a decrease in the density increment is observed for decreasing protein size. A simple model is proposed in which the basic idea is to approximate the protein molecule as an effective ellipsoid and to partition the relevant parameters, i.e. the solvent-accessible volume and the corresponding solvent density, into two regions: inside and outside the effective protein ellipsoid. It is found that, within the model developed here, almost all of the hydration-density increase is located inside the protein ellipsoid, basically corresponding to pockets within, or at the surface of the protein molecule. The observed decrease in the density increment is caused by the protein size only and no difference is found between antifreeze and non-antifreeze proteins.
- This article is part of the themed collection: Coronavirus articles - free to access collection


 Please wait while we load your content...
Please wait while we load your content...