Non-humidified fuel cells using a deep eutectic solvent (DES) as the electrolyte within a polymer electrolyte membrane (PEM): the effect of water and counterions
Abstract
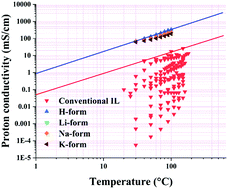
In this research, deep eutectic solvents (DESs) were prepared and employed as the electrolyte in Nafion membranes. Different factors, such as the water content and Nafion counterions (H+, Li+, Na+ and K+), which could influence the PEM performance, were evaluated. The obtained results showed that the presence of water may have a constructive or destructive effect on the DES and Nafion/DES properties, which should be considered for their final applications. Also, the electronegativity of the counterion can significantly influence the Nafion/DES proton conductivity. The prepared Nafion/DES composite membranes showed superconducting properties as a result of a Grotthuss-like mechanism for proton conduction. The conductivities of the prepared membranes were compared to those of other membranes based on an upper bound concept, which showed the potential use of DESs as a promising alternative to conventional ionic liquids. Finally, the fuel cell performances of the prepared membranes at different temperatures were evaluated.


 Please wait while we load your content...
Please wait while we load your content...