On the microscopic origin of the cryoprotective effect in lysine solutions†
Abstract
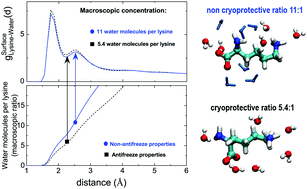
The amino acid lysine has been shown to prevent water crystallization at low temperatures in saturated aqueous solutions [S. Cerveny and J. Swenson, Phys. Chem. Chem. Phys., 2014, 16, 22382–22390]. Here, we investigate two ratios of water and lysine (5.4 water molecules per lysine (saturated) and 11 water molecules per lysine) by means of the complementary use of computer simulations and neutron diffraction. By performing a detailed structural analysis we have been able to explain the anti-freeze properties of lysine by the strong hydrogen bond interactions of interstitial water molecules with lysine that prevent them from forming crystalline seeds. Additional water molecules beyond the 1 : 5.4 proportion are no longer tightly bonded to lysine and therefore are free to form crystals.


 Please wait while we load your content...
Please wait while we load your content...