A study on the effects of mixed organic cations on the structure and properties in lead halide perovskites†
Abstract
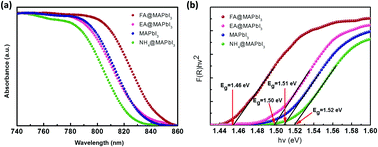
Recently, organic-lead halide perovskites have emerged as strong competitors in photovoltaic and general optoelectronic applications owing to their remarkable characteristics, including high balance hole and electron mobility, strong absorption coefficient and long carrier lifetime. However, the commercial applicability of these materials is hampered by their relative lack of stability compared to established inorganic and organic semiconductors. It has been found that it is possible to tune the properties and stability of the organic-lead halide perovskite materials by site-substitution at A sites of the ABX3 perovskite structure. Here, organic cations (NH4+, HC (NH2)2+, and CH3CH2NH3+) were successfully incorporated in the methylammonium-based perovskite crystal to investigate the role of organic cation size on structure, optical features, thermal stability, and electrical transport properties. Powder X-ray diffraction results indicate that the size of organic cations can not only cause lattice strain by lattice contraction or dilation but also may induce phase transitions by octahedral tilting. Meanwhile, band gaps of these crystals show that organic cations could tune the band gap energy of the perovskites by changing the Pb–I bond angle, which agrees with previous reports. The result of thermogravimetric analysis indicates that thermal stability is related to the probability of HI formation, which is directly related to the acidity of the organic species. These results represent an important step to highlight the role of organic cations in hybrid perovskite materials, which will further benefit the fundamental understanding of materials and device optimization.


 Please wait while we load your content...
Please wait while we load your content...