Influence of nonmetal dopants on charge separation of graphitic carbon nitride by time-dependent density functional theory†
Abstract
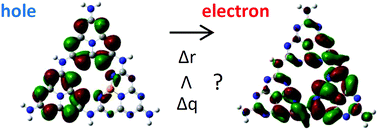
Photocatalysts are crucial materials for green energy production and environmental remediation. Nonmetal-doped graphitic carbon nitride (g-C3N4) has attracted much attention in recent years because of its low-cost and desired photocatalytic performances, such as a high charge separation efficiency and broad visible light absorption. In this study, we conducted time-dependent density functional theory calculations, and a wavefunction analysis to evaluate the charge separation characteristics of phosphorus-, oxygen- and sulfur-doped g-C3N4 upon photo-excitation. In particular, we examined the electron–hole pair distances, the electron–hole pair overlaps, and the amounts of transferred charge. The phosphorus, oxygen, and sulfur dopants shifted the lowest unoccupied molecular orbital of doped heptazine rings downward to facilitate the electron transfer upon photo-excitation. Generally, the phosphorus dopant triggers relatively high amounts of transferred charge, strong electron–hole pair separations, and low electron–hole overlaps compared to oxygen and sulfur dopants. At a low dopant concentration, the sulfur dopant showed a similar effect to that of the phosphorus dopant. The phosphorus dopants not only contributed to the electron–hole pair separation, but also attracted photo-excited electrons. The comparison of different dopant distributions on the heptazine rings of g-C3N4 showed that dopants concentrated on one heptazine ring exhibit better charge separation performance than dopants dispersed on different heptazine rings do. This indicates that the doping configuration has a stronger effect than the doping concentration on the charge separation efficiency in nonmetal-doped g-C3N4.


 Please wait while we load your content...
Please wait while we load your content...