Lithium migration pathways at the composite interface of LiBH4 and two-dimensional MoS2 enabling superior ionic conductivity at room temperature†
Abstract
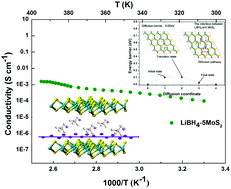
LiBH4 is one of the most promising solid electrolyte materials for use in solid-state batteries because its hexagonal phase above 110 °C offers Li-ion conductivity of almost 10−3 S cm−1. However, near room temperature, its orthorhombic phase delivers Li-ion conductivity of only 10−8 S cm−1, which considerably hampers its further applications. In the present study, a highly disordered interface between LiBH4 and two-dimensional MoS2 in the composite material was formed, yielding ionic conductivity of 10−4 S cm−1 at room temperature. LiBH4 and MoS2 are found to be in close contact without the formation of any intermediate phase at the interface. First-principles calculations employing density functional theory (DFT) and the nudged elastic band (NEB) method reveal that the migration energy barrier on three specific pathways could be established via microstructure analyses. It was found that the interface between the two phases yields the lowest Li-ion diffusion barrier among all the possible Li-ion pathways; further, the superior conductivity of the composite could be attributed to the interface with high Li-ion conductivity. This study proposes a new strategy for designing solid electrolytes and provides certain possibilities for two-dimensional materials to serve as superior solid electrolytes.


 Please wait while we load your content...
Please wait while we load your content...