An insight into the corrosion of alkali aluminoborosilicate glasses in acidic environments†
Abstract
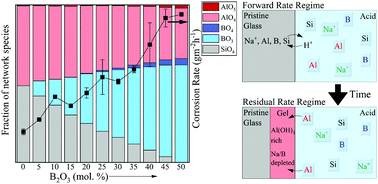
The majority of the literature on glass corrosion focuses on understanding the dissolution kinetics and mechanisms of silicate glass chemistries in the neutral-to-alkaline aqueous regime owing to its relevance in the fields of nuclear waste immobilization and biomaterials. However, understanding the corrosion of silicate-based glass chemistries over a broad composition space in the acidic pH regime is essential for glass packaging and touch screen electronic display industries. A thorough literature review on this topic reveals only a handful of studies that discuss acid corrosion of silicate glasses and their derivatives—these include only a narrow set of silicate-based glass chemistries. Although the current literature successfully explains the dissolution kinetics of glasses based upon classically understood aqueous corrosion mechanisms, more recent advancements in atomic-scale characterization techniques, have enabled a better understanding of reactions taking place directly at the pristine glass–fluid interface which has facilitated the development of a unifying model describing corrosion behavior of silicate glasses. Based on the corrosion mechanisms described and the questions raised in preceding literature, the present study focuses on understanding the corrosion mechanisms governing metaluminous (Na/Al = 1) sodium aluminoborosilicate glasses in acidic environments across a wide composition-space (ranging from SiO2-rich to B2O3-rich compositions), with particular emphasis on understanding the reactions taking place near the glass–fluid interface. Using state-of-the-art characterization techniques including nuclear magnetic resonance (NMR) spectroscopy, Rutherford backscattering, X-ray photoelectron spectroscopy (XPS) and elastic recoil detection analysis (ERDA), it has been shown that stepwise B2O3 substitutions into nepheline (NaAlSiO4) glass, although causing non-linear changes in glass structure network structural features, leads to strikingly linear increases in the forward dissolution rate at pH = 2. While the glasses undergo congruent dissolution in the forward rate regime, the residual rate regime displays evidence of preferential extraction near the glass surface (i.e., enrichment in aluminum content upon corrosion through AlO4 → Al(OH)3 evolution) implying that dissolution–re-precipitation processes may occur at the glass–fluid interface in both B2O3-rich and SiO2-rich glass compositions—albeit with vastly dissimilar reaction kinetics.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...