Impact of the reaction pathway on the final product in on-surface synthesis†
Abstract
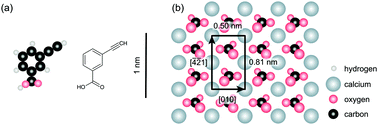
On-surface synthesis provides a very promising strategy for creating stable functional structures on surfaces. In the past, classical reactions known from solution synthesis have been successfully transferred onto a surface. Due to the presence of the surface, on-surface synthesis provides the potential of directing the reaction pathway in a manner that might not be accessible in classical solution synthesis. In this work, we present evidence for an acetylene polymerization from a terminal alkyne monomer deposited onto calcite (10.4). Strikingly, although the dimer forms on the surface as well, we find no indication for diacetylene polymerization. This is in sharp contrast to what is observed when directly depositing the dimers on the surface. The different pathways are linked to the specific arrangement of the dimers on the surface. When forming stripes along the [−4−21] direction, the diacetylene polymerization is prohibited, while when arranged in stripes aligned along the [010] direction, the dimers can undergo diacetylene polymerization. Our work thus constitutes a demonstration for controlling the specific reaction pathway in on-surface synthesis by the presence of the surface.
- This article is part of the themed collection: 2020 PCCP HOT Articles

 Please wait while we load your content...
Please wait while we load your content...