Active centre and reactivity descriptor of a green single component imidazole catalyst for acetylene hydrochlorination†
Abstract
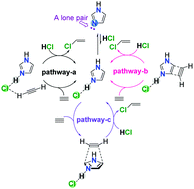
A green catalyst for acetylene hydrochlorination yielding a VCM is presented using imidazole as a single component metal-free catalyst. The mechanisms and reactivities of imidazole-catalyzed acetylene hydrochlorination have been investigated by combined computational and experimental studies. The electronic effects of ortho-substituents on the reactivities have also been investigated. Through theoretical calculations and experimental studies, the nitrogen-atom including a lone pair active site of single component imidazole for metal-free acetylene hydrochlorination is proposed. It is suggested that the nitrogen-atom including a lone pair of imidazole adsorbs an HCl molecule to form an imidazole-HCl complex, which serves as the active catalyst to participate in the reaction process of acetylene hydrochlorination. Besides, the results show that C2H2 assists in the electrophilic addition of HCl, undergoing an almost planar six-membered ring transition state. Computational studies on the ortho-substitution of the active sites will have an important impact on the catalytic efficiency.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...