Translocation of a hydroxyl functionalized carbon dot across a lipid bilayer: an all-atom molecular dynamics simulation study†
Abstract
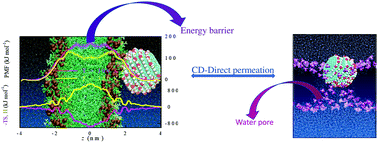
The major applications of carbon dots (CDs) (e.g. bio-imaging and targeted drug delivery) necessitate the latter to permeate across the lipid bilayer membrane. Unfortunately, the mechanism of permeation is poorly understood. Between the two possible routes for permeation of a nanoparticle like CDs—an endocytic pathway and direct passive permeation—the endocytic path is known to be more common, despite the fact that the passive permeation is preferred over the endocytosis for targeted drug delivery. Here, we have focused on the direct permeation of a hydroxyl functionalized CD across the POPC lipid bilayer membrane using all-atom MD simulations. We have estimated the free energy profile for the translocation of the CD across the lipid bilayer, with a barrier height of ∼170 kJ mol−1 situated at the lipid bilayer center (z = 0 nm). Using the free energy profile, we have calculated a negligible permeability coefficient value, which strongly suggests that it is almost impossible for a CD to penetrate directly across the lipid bilayer. The possible impact on the lipid bilayer structure by the CD is also investigated. Although the CD does not affect the bilayer structure up to a certain degree of penetration, the impact increases substantially when entered into the bilayer interior.

 Please wait while we load your content...
Please wait while we load your content...