Reactions of a distonic peroxyl radical anion influenced by SOMO–HOMO conversion: an example of anion-directed channel switching†
Abstract
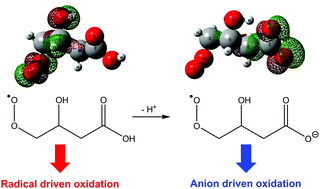
In free radicals the singly occupied molecular orbital (SOMO) typically has the highest energy. Recent examples of distonic radical anions were found, however, to disobey the usual orbital configuration, with the singly occupied molecular orbital buried energetically underneath doubly occupied orbitals. This unusual ordering of electrons, which contradicts the aufbau principle, has been characterized as SOMO–HOMO orbital conversion and is expected to perturb radical anion reactivity by branching toward anion-driven over radical-driven processes. Here, we use ion trap mass spectrometry and ab initio calculations to demonstrate that SOMO–HOMO orbital conversion influences the reactivity of a distonic peroxyl radical anion. Experimentally, we generated a distonic radical anion of β-hydroxy glutaric acid, ˙CH2CH(OH)CH2C(O)O−, and investigated its subsequent reaction with O2 in the gas phase. Theoretical calculations predict that reactions proceed through five isomeric C4H6O5˙− intermediates, two of which exhibit SOMO–HOMO conversion. The detected product ions, corresponding to loss of ˙OH + CO2, ˙OH + HCHO, HO2˙, and HO2˙ + CO2 from the peroxyl radical, can all be reconciled by the proposed reaction mechanism. Finally, we compare the oxygen recombination reaction of the distonic radical ion to the corresponding neutral radical (i.e., ˙CH2CH(OH)CH2C(O)OH). These calculations demonstrate that SOMO–HOMO conversion results in channel switching in the distonic radical anion, suppressing radical-driven mechanisms and promoting pathways that directly involve the anion site.


 Please wait while we load your content...
Please wait while we load your content...