Thickness dependent homogeneous crystallization of ultrathin amorphous solid water films†
Abstract
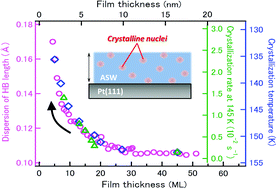
The crystallization mechanism and kinetics of amorphous materials are of paramount importance not only in basic science but also in the application field because they are closely related to their thermal stability. In the case of amorphous nanomaterials, thermal stability distinctively different from that of bulk materials often emerges. Despite intensive studies in the past, a thorough understanding of the stability at the molecular level has not been reached particularly on how crystallization processes depend on size and are influenced by their surface and interface. In this article, we report the film-size-dependent crystallization of thermally relaxed nonporous ASW ultrathin films on a Pt(111) surface as a benchmark system of amorphous molecular films. The crystallization processes at the surface and interior of the ASW ultrathin films are monitored simultaneously with thermal desorption and infrared reflection absorption, respectively, as a function of the film thickness. Here, we demonstrate that the crystallization is initiated solely by “homogeneous nucleation” irrespective of the film thickness while the crystallization rate remarkably depends on the thickness; the rate of 5-layer (∼1.5 nm) ASW films is one order of magnitude higher than that of 20-layer (∼6 nm) films. Moreover, we found a clear correlation between the film-thickness-dependent crystallization kinetics and microscopic structural disorder associated with the broad distribution of hydrogen-bond lengths between water molecules.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...