CO2 activation through C–N, C–O and C–C bond formation†
Abstract
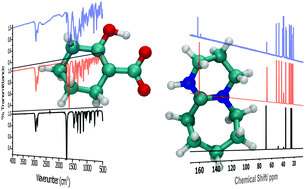
A comparative model for the chemisorption of CO2 was explored via three representative reaction pathways: carboxylation of cyclohexanone, carbonation of cyclohexanol, and carbamation of cyclohexylamine. The model substrates were activated using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, an amidine superbase). For each of these reactions, the formation of the corresponding CO2 adducts was confirmed by 13C nuclear magnetic resonance and Fourier-transform infrared spectroscopy measurements. It was demonstrated that CO2 fixation occurred through either an enol-CO2 adduct (i.e. carboxylation), proton shuttling process (i.e. carbonation), or self-activation mechanism (i.e. carbamation). Volumetric adsorption measurements indicated that cyclohexanol was superior in its uptake capacity (11.7 mmol CO2 g−1 sorbent) in comparison to cyclohexylamine (9.3 mmol CO2 g−1 sorbent) or cyclohexanone (8.5 mmol CO2 g−1 sorbent). As supported by density functional theory calculations, this trend was expected given the fact that the carbonation reaction proceeded through a more thermodynamically favorable reaction process.


 Please wait while we load your content...
Please wait while we load your content...