ONIOM investigations of the heme degradation mechanism by MhuD: the critical function of heme ruffling†
Abstract
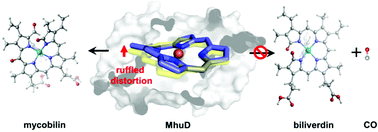
The oxygen-dependent heme utilization degrading enzyme in Mycobacterium tuberculosis (MhuD) uniquely integrates monooxygenase and dioxygenase functions in a single active site. It cannot convert heme to biliverdin as canonical heme oxygenases but generates mycobilin without releasing carbon monoxide. Herein, by employing ONIOM calculations, we investigated the heme degradation mechanism of MhuD. Our calculations revealed that MhuD firstly follows a canonical monooxygenation mechanism to hydroxylate heme on the δ-meso carbon guided by the asparagine residue Asn7, which experiences a 21.2 kcal mol−1 energy barrier in the O–O cleavage rate-limiting step during the conversion process from ferric heme-hydroperoxy species to mycobilin. In the second degradation step, the ruffled conformation of oxoheme (oxoheme is the ferrous π radical complex formed by hydroxyheme experiencing deprotonation in the hydroxyl group and intramolecular electron transfer) imposed by the hydrophobic environment of the enzyme not only inhibits the continuing conversion of oxoheme to biliverdin but also endows the meso-carbons with radical characteristics, which turns the second degradation step to a dioxygenation reaction with 20.4 kcal mol−1 energy barrier. We further analysed the electronic structure change along the reaction process. Our calculation discovered that the ruffled structure of oxoheme is critical to the regiospecificity and even atom location selectivity, as well as the reaction mechanism of the degradation process.


 Please wait while we load your content...
Please wait while we load your content...