Mechanism of Mg extraction from MgMn2O4 during acid digestion†
Abstract
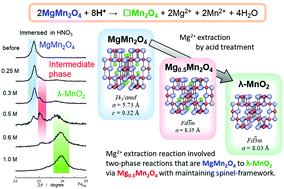
MgMn2O4 having a spinel structure is a very attractive material for the positive electrode in Mg-ion batteries, since its reversible Mg extraction/insertion reaction can lead to a large reversible capacity. While the Mg extraction from MgMn2O4 has been reported, the reaction mechanism remains unclear. In this paper, Mg ions were chemically extracted from MgMn2O4 by acid digestion at various concentrations to produce MgxMn2O4 (0 < x < 1). The results showed that Mg extraction from MgMn2O4 is a two-step two-phase reaction, via the intermediate Mg0.5Mn2O4 to the fully oxidised Mn2O4. The kinetics of Mg extraction was clarified using acid digestion experiments of different durations, and the direct reaction pathway of MgMn2O4 oxidation to λ-MnO2 was the fastest process. This may explain the difficulty in Mg extraction from MgMn2O4 using electrochemical methods.


 Please wait while we load your content...
Please wait while we load your content...