Energetics of paramagnetic oxide clusters: the Fe(iii) oxyhydroxy Keggin ion†
Abstract
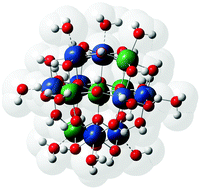
The energetics of the different spin states of the five Baker–Figgis isomers of the iron(III) Keggin ion, [Fe(O4)(Fe(OH)2(OH2))12]7+, have been investigated using density functional theory in order to demonstrate how the energy landscape of medium-to-large discrete paramagnetic transition metal oxide clusters with large numbers of antiferromagnetically coupled centres can be resolved. Antiferromagnetic coupling causes the energies to span a surprisingly large range of 30 kcal mol−1, as determined by calculating the energies of all 664 unique spin configurations based on determination of the antiferromagnetic coupling constants by density functional theory. A program which simplifies the resolution of the energetics of this type of system is also provided.


 Please wait while we load your content...
Please wait while we load your content...