Micro-kinetic model of electrochemical carbon dioxide reduction over platinum in non-aqueous solvents†
Abstract
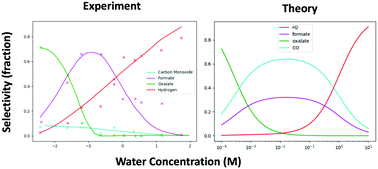
The competition between the hydrogen evolution reaction and the electrochemical reduction of carbon dioxide to multi-carbon products is a well-known challenge. In this study, we present a simple micro-kinetic model of these competing reactions over a platinum catalyst under a strong reducing potential at varying proton concentrations in a non-aqueous solvent. The model provides some insight into the mechanism of reaction and suggests that low proton concentration and a high fraction of stepped sites is likely to improve selectivity to multi-carbon products.


 Please wait while we load your content...
Please wait while we load your content...