Pressure-stabilized polymerization of nitrogen in alkaline-earth-metal strontium nitrides†
Abstract
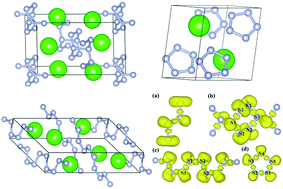
High-pressure technology can help us to obtain excellent materials. We have explored alkaline-earth-metal strontium nitrides under different pressures, theoretically. A variety of stable Sr–N structures were predicted by the structure searching method using CALYPSO code. Six new stoichiometries, SrN, Sr2N3, SrN2, SrN3, SrN4, and SrN5, were predicted. And our calculation proved that all these compounds were stable existing under ambient pressure up to 100 GPa. A rich variety of poly-nitrogen forms appeared in the newly predicted SrNx compounds, including four nitrogen polymerization forms: ranging from N2, N3, N4, and N5 molecules, to zig-zag nitrogen chains and extended chains connected by puckered “N6” rings. Significantly, the 1D extended polymeric chain of puckered “N6” rings was firstly identified in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) -SrN3 structure at 60 GPa. Another N-rich C2/c-SrN4 was stable only under the relatively high-pressure of 20 GPa, but this phase can be quenched under atmospheric pressure. The N-rich phase SrN5 maintained structural stability when the pressure reached 50–70 GPa. The delocalization of π electrons from N atoms was the principal cause for its metallicity in SrN5. In this paper, our calculated results indicated that the energetic poly-nitrides in alkaline-earth-metal nitrides can be obtained by the high-pressure method.
-SrN3 structure at 60 GPa. Another N-rich C2/c-SrN4 was stable only under the relatively high-pressure of 20 GPa, but this phase can be quenched under atmospheric pressure. The N-rich phase SrN5 maintained structural stability when the pressure reached 50–70 GPa. The delocalization of π electrons from N atoms was the principal cause for its metallicity in SrN5. In this paper, our calculated results indicated that the energetic poly-nitrides in alkaline-earth-metal nitrides can be obtained by the high-pressure method.


 Please wait while we load your content...
Please wait while we load your content...