Mechanism of photochromic transformations and photodegradation of an asymmetrical 2,3-diarylcyclopentenone†
Abstract
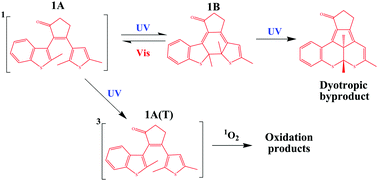
A mechanistic study of the photochromic properties and photodegradation processes of an asymmetrical diarylcyclopentenone bearing thiophene and benzothiophene units using stationary photolysis, nanosecond laser flash photolysis and time-resolved luminescence was performed. It was found that the light-induced reversible isomerization of (3-(2,5-dimethyltiophen-3-il)-2-(2-methyl-1-benzylthiophen-3-il)cyclopent-2-en-1-one, compound 1) from open to closed form is a common photochromic transformation inherent to diarylethenes, while the photodegradation process proceeds in two ways. The first is a formal 1,2-dyotropic rearrangement, proceeding without the participation of oxygen. The second is the oxygen-dependent mechanism involving the excitation of the open form 1A into the triplet state, quenching of the latter by dissolved oxygen, and oxidation of the initial compound by singlet oxygen.


 Please wait while we load your content...
Please wait while we load your content...