Low temperature dielectric properties and NTCR behavior of the BaFe0.5Nb0.5O3 double perovskite ceramic
Abstract
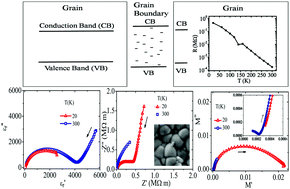
The double perovskite-structured barium iron niobate (BaFe0.5Nb0.5O3, BFN), a lead-free ferroelectric, shows a very high dielectric constant value. We report here the temperature dependent dielectric permittivity, impedance and electric modulus behavior of the BFN ceramic studied between 300 K and 20 K. The phase-pure BFN powder sample was synthesized using a conventional solid state reaction route. The microstructure and the temperature dependent dielectric behavior of the sample are correlated. The dipolar and Maxwell–Wagner (M–W) polarizations of the sample show distinctly different relaxation behaviors at the grain and grain boundary (GB) regions. With a decrease in temperature from 300 K to 20 K, the M–W polarization contribution decreases monotonously. Furthermore, due to the ion-blocking effect originating from the atomic disorder at the GB regions, the impedance of the GB is found to be higher than that of the grains. The grains and GB regions were found to have characteristically very different dipolar relaxation times. Interestingly, the dipolar relaxation time distribution is the broadest at 120 K due to the temperature dependent shift of the dipolar relaxation times at the grain and GB regions. Furthermore, our results confirm that the dipolar polarization mechanism in BFN is associated only with short-range movement of the cations. Our systematic investigation provides a detailed understanding of the low temperature dielectric properties of the BaFe0.5Nb0.5O3 ceramic and makes it potentially attractive for low temperature thermistor applications.


 Please wait while we load your content...
Please wait while we load your content...