A theoretical study on Na+ solvation in carbonate ester and ether solvents for sodium-ion batteries†
Abstract
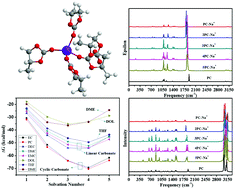
The electrochemical performance of sodium-ion batteries is strongly related to the electrolyte solvents. Na+ solvation in commonly used carbonate esters such as ethylene carbonate (EC), propylene carbonate (PC), diethyl carbonate (DEC), dimethyl carbonate (DMC), and ethyl methyl carbonate (EMC) as well as in ether solvents such as 1,3-dioxolane (DOL), tetrahydrofuran (THF), and dimethoxyethane (DME) is studied by the density functional theory for sodium-ion batteries. It is indicated that the thermodynamic equilibrium is reached when forming 4sol–Na+ in the EC, PC, DMC, EMC, DEC, and THF solvents by spontaneous stepwise solvation reactions, and the formation of 3sol–Na+ complexes will reach thermodynamic equilibrium in DOL and DME at room temperature. It is demonstrated that Na+ is more easily solvated by the carbonate ester-based solvents EC, PC, DEC, DMC and EMC compared with that for the ether-based solvents DOL, THF and DME. In addition, the cyclic carbonate ester solvents more easily form a solvation–Na+ complex compared with the linear carbonate ester solvents, and THF is the easiest to form the solvation–Na+ complex among the three ether-based solvents. It is also indicated that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and carbonate C–O bond stretching vibrations in carbonate ester solvation complexes move to higher and lower frequencies, respectively, with the decrease in Na+ concentration. In addition, the C–O stretching vibrations with or without Na+ interactions in the ether solvation complexes shift to higher and lower frequencies, respectively, and the shift in frequency is not obvious after forming the maximum innermost solvation shell.
O and carbonate C–O bond stretching vibrations in carbonate ester solvation complexes move to higher and lower frequencies, respectively, with the decrease in Na+ concentration. In addition, the C–O stretching vibrations with or without Na+ interactions in the ether solvation complexes shift to higher and lower frequencies, respectively, and the shift in frequency is not obvious after forming the maximum innermost solvation shell.


 Please wait while we load your content...
Please wait while we load your content...