Iodide conversion to iodate in aqueous and solid aerosols exposed to ozone†
Abstract
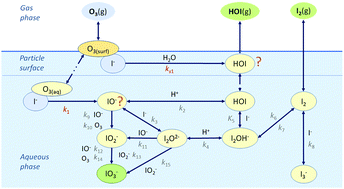
The aqueous-phase and surface reactions of ozone (O3) with iodide (I−) in/on seawater have been recently found to be a strong atmospheric source of iodine. In addition, ozone also reacts with I− in solid and aqueous sea-salt aerosol. However, the primary products of the heterogeneous reactions of ozone with I− have not been clarified. In this paper, solid and aqueous KI aerosols have been exposed to ozone in an aerosol flow tube system and I− and iodate (IO3−) concentrations have been measured by UV-Vis spectroscopy. The results of these experiments have been combined with a kinetic model to elucidate the primary products of the aqueous and surface reactions. The reaction of ozone with aqueous iodide has been inferred to originate different products depending on whether it occurs at the surface via O3 adsorption (product I2−) or in the aqueous phase via O3 solvation (product IO−). The surface reaction of ozone with solid KI in the presence of water vapor forms KIO3, and other species, which are likely to be gaseous. Although the reactions have been studied in aerosols, the results can be extrapolated to aqueous solutions as well.


 Please wait while we load your content...
Please wait while we load your content...