Quantitative analysis of the coupling between proton and electron transport in peptide/manganese oxide hybrid films†
Abstract
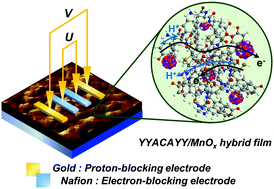
Understanding how electrons and protons move in a coupled manner and affect one another is important to the design of proton–electron conductors and achieving biological transport in synthetic materials. In this study, a new methodology is proposed that allows for the quantification of the degree of coupling between electrons and protons in tyrosine-rich peptides and metal oxide hybrid films at room temperature under a voltage bias. This approach is developed according to the Onsager principle, which has been thoroughly established for the investigation of mixed ion-electron conductors with electron and oxide ion vacancies as carriers at high temperatures. Herein, a new device platform using electron-blocking electrodes provides a new strategy to investigate the coupling of protons and electrons in bulk materials beyond the molecular level investigation of coupled proton and electron transfer. Two Onsager transport parameters, αi* and σe′, are obtained from the device, and the results of these transport parameters demonstrate that the coupled transport of electrons and protons inside the hybrid film plays an important role in the macroscopic-scale conduction. The results suggest that an average of one electron is dragged by one proton in the absence of a direct driving force for electron movement ∇ηe.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...