On the stability of hydroxyl groups on substituted titania
Abstract
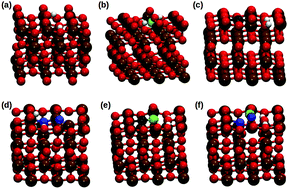
The present study reports the stability of hydroxyl groups involving the surface coordinated oxygens of Pd,C,N-doped, and Pd/C and Pd/N-codoped anatase TiO2, probed using DFT calculations. Two unique surface planes, (001) and (100), were chosen for the analysis of the stability of hydroxyl groups and their activities were studied by net oxygen activation analysis. The hydroxyl group formation energies ranged between −6.16 and −7.88 eV for the C,N-doped, and Pd/C and Pd/N-codoped TiO2(001) and (100) planes. The order of hydroxyl stability was observed to be TiO2(001) > TiO2(100) > Pd/C-codoped (001) > Pd/N-codoped (100) > Pd/C-codoped (100) > N-doped (001) > C-doped (100) > N-doped (100) > Pd/N-codoped (001) > C-doped (001) planes of TiO2. Although the formation energies of Pd/C and Pd/N-codoped TiO2 were marginally higher compared to those of the pure TiO2(001) and (100) planes, they exhibited a higher net oxygen activation of 32.1% and 28.9% over the surface exposed (100) plane, which indicated the better feasibility of reversible exchange of lattice oxygen. Electron density maps displayed the surface reconstruction phenomenon corresponding to the rearrangement of surface atoms and the transfer of electrons between O–H over the (001) and (100) planes of C,N-doped, and Pd/C and Pd/N-codoped TiO2.


 Please wait while we load your content...
Please wait while we load your content...