Entropic restrictions control the electric conductance of superprotonic ionic solids†
Abstract
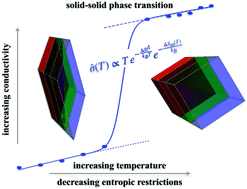
The crystallographic structure of solid electrolytes and other materials determines the protonic conductivity in devices such as fuel cells, ionic-conductors, and supercapacitors. Experiments show that a rise of the temperature in a narrow interval may lead to a sudden increase of several orders of magnitude of the conductivity of some materials, a process called a superprotonic transition. Here, we use a novel macro-transport theory for irregular domains to show that the change of entropic restrictions associated with solid–solid phase or structural transitions controls the sudden change of the ionic conductivity when the superprotonic transition takes place. Specifically, we deduce a general formula for the temperature dependence on the ionic conductivity that fits remarkably well experimental data of superprotonic transitions in doped cesium phosphates and other materials reported in the literature.


 Please wait while we load your content...
Please wait while we load your content...