In situ structural kinetics of picosecond laser-induced heating and fragmentation of colloidal gold spheres†
Abstract
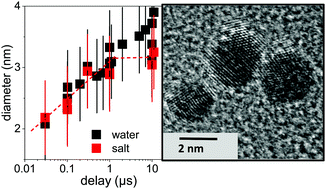
Fragmentation of colloidal 54 nm gold nanoparticles by picosecond laser pulses is recorded by time-resolved X-ray scattering, giving access to structural dynamics down to a 80 ps resolution. Lattice temperature and energy dissipation have been quantified to verify that the maximum applied fluence of 1800 J m−2 heats up the particles close to boiling. Already within 30 ns, particles with significantly lower particle sizes of 2 to 3 nm are detected, which hints towards an ultrafast process either by a thermal phase explosion or Coulomb instability. An arrested growth is observed on a microsecond time scale resulting in a final particle size of 3–4 nm with high yield. In this context, the fragmentation in a NaCl/NaOH solution seems to limit growth by electrostatic stabilization of fragments, whereas it does not modify the initial product sizes. The laser-induced fragmentation process is identified as a single-step, instantaneous reaction.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...