Theoretical insights into the effect of the overpotential on CO electroreduction mechanisms on Cu(111): regulation and application of electrode potentials from a CO coverage-dependent electrochemical model†
Abstract
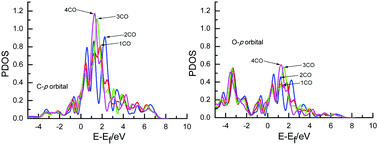
A recently proposed CO coverage-dependent electrochemical model combined with the calculation of electronic structure is applied for the first time to study the effect of the overpotential on Cu-catalyzed CO electroreduction mechanisms by changing the coverage of surface adsorbed CO. The results show that the presently defined CH2O and CHOH pathways may be able to occur parallelly under different overpotentials. However, high overpotentials will facilitate CO electroreduction, thus explaining why a high overpotential is required during CO2 electroreduction in experiments on Cu electrodes. The potential-limiting step may be further CO electroreduction into CHO, which is considered as the origin of the experimentally observed high overpotentials. The analyses of electronic structure show that an adsorbed COδ− species is formed on the Cu electrodes, validating the previous experimental speculations on electron transfer between CO and Cu electrodes. More and more electrons are transferred into the π antibonding orbitals of the adsorbed CO with increasing surface CO coverage, leading to increasing overpotential and weaker and weaker CO bonding with the Cu surface. Thus, the significantly lower barrier of further CO electroreduction at higher overpotential can be correlated with lower CO adsorption energy. Interestingly, it is found that there is greater localization of electrons around the C than the O atom in the adsorbed CO molecule, explaining why the hydrated proton prefers to reach the C atom to form intermediate CHO rather than COH.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...