First-principles study on the mechanism of photocatalytic reduction of nitrobenzene on the rutile TiO2(110) surface†
Abstract
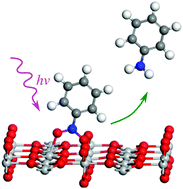
Photocatalytic synthesis of organic compounds has attracted more and more attention recently. In this work, we present a theoretical study on the molecular mechanism of the photocatalytic reduction of nitrobenzene to aniline on the rutile TiO2(110) surface. We have studied the adsorption and conversion of nitrobenzene at both the surface Ti site and the oxygen vacancy (Ov) site. The full reaction pathways at these two sites were calculated. The rate-limiting step and possible intermediates were identified. The results suggest that Ov is more active in the adsorption and conversion of nitrobenzene. Interestingly, we found that the chemistry of nitrobenzene on the rutile TiO2(110) surface, especially the breaking of the N–O bond, is closely related to the number of excess electrons available. Based on the calculation, we have proposed a full molecular mechanism which is compatible with the existing experiments. The results should be helpful for the design of more efficient photocatalysts for the conversion of nitrobenzene.


 Please wait while we load your content...
Please wait while we load your content...