Equilibrium structures of water molecules confined within a multiply connected carbon nanotube: a molecular dynamics study†
Abstract
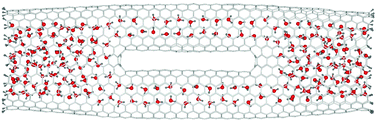
Water confinement inside a carbon nanotube (CNT) has been one of the most exciting subjects of both experimental and theoretical interest. Most of the previous studies, however, considered CNT structures with simple cylindrical shapes. In this paper, we report a classical molecular dynamics study of the equilibrium structural arrangement of water molecules confined in a multiply connected carbon nanotube (MCCNT) containing two Y-junctions. We investigate the structural arrangement of the water molecules in the MCCNT in terms of the density of water molecules and the average number of hydrogen bonds per water molecule. Our results show that the structural rearrangement of the H2O molecules takes place several angstroms ahead of the Y-junction, rather than only at the CNT junction itself. This phenomenon arises because it is difficult to match the boundary condition for hydrogen bonding in the region where two different hydrogen-bonded structures are interconnected with each other.


 Please wait while we load your content...
Please wait while we load your content...