Thermal stability modulation of the native and chemically-unfolded state of bovine serum albumin by amino acids†
Abstract
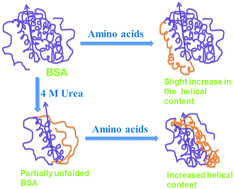
Cells are crowded with various cosolutes including salts, osmolytes, nucleic acids, peptides and proteins. These cosolutes modulate the protein folding equilibrium in different ways, however, a unifying concept remains elusive. To elucidate the cosolute size-effect, macromolecular crowders are commonly compared to their monomeric building blocks (e.g. dextran vs. glucose or polyethylene glycol with different degrees of polymerization). To the best of our knowledge, such studies do not exist for protein crowders, raising the question of how single amino acids modulate the folding equilibrium. Therefore, we investigate the effect of glycine, alanine, proline and arginine on the stability of a model globular protein bovine serum albumin (BSA) upon thermal and urea-induced unfolding. We use three complementary techniques, fluorescence spectroscopy (as a local site-specific probe), circular dichroism (as a global probe for α-helical structure) and differential scanning calorimetry (to probe the energetics of unfolding). We find that the amino acids modulate BSA stability and unfolding, however, without following a particular trend with either the hydrophobicity scale or the solvent accessible surface area (SASA) of the added amino acids. Our data rather suggest that solvation effects play a role in understanding the cosolute effect.


 Please wait while we load your content...
Please wait while we load your content...