Amorphous tungsten phosphosulphide-modified CdS nanorods as a highly efficient electron-cocatalyst for enhanced photocatalytic hydrogen production
Abstract
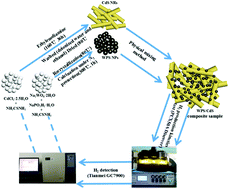
Improving the utilization rate of photogenerated electrons generated by visible light excitation is an important factor to improve the activity of photocatalytic decomposition of water for hydrogen evolution. In this study, amorphous tungsten phosphosulphide nanoparticle (WPS NP)-modified CdS (WPS/CdS) catalysts were successfully prepared by a simple physical mixing method. The activity of the 15% WPS/CdS composite catalyst is the best, and the average hydrogen production rate reached 123 257 μmol g−1 in 5 h, and the highest AQE of 9.15% is derived at 420 nm for the 15% WPS/CdS composite catalyst. Simultaneously, five cycle stability tests were performed on the 15% WPS/CdS composite catalyst, and the results show that the 15% WPS/CdS composite catalyst exhibits a high stability. WPS NPs not only improve the visible light absorption rate, but also provide a large number of exposed active sites for the hydrogen evolution reaction. These active sites can capture photogenerated electrons on CdS NRs quickly, and can be used for the hydrogen evolution reaction quickly, promoting the transmission and separation of photogenerated charges and inhibiting the recombination of photogenerated electron and hole pairs. Thus, the utilization rate of photogenerated electrons generated by visible light is improved, and the activity of the photocatalyst is significantly increased.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...