Mechanistic study of the ATP hydrolysis reaction in dynein motor protein†
Abstract
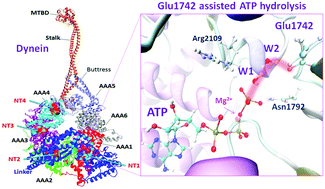
Dynein, a large and complex motor protein, harnesses energy from adenosine triphosphate (ATP) hydrolysis to regulate essential cellular activities. The ATP hydrolysis mechanism for the dynein motor is still shrouded in mystery. Herein, molecular dynamics simulations of a dynein motor disclosed that two water molecules are present close to the γ-phosphate of ATP and Glu1742 at the AAA1 site of dynein. We have proposed three possible mechanisms for the ATP hydrolysis. We divulge by using a quantum mechanics/molecular mechanics (QM/MM) study that two water molecules and Glu1742 are crucial for facilitating the ATP hydrolysis reaction in dynein. Moreover, the ATP hydrolysis step is initiated by the activation of lytic water (W1) by Glu1742 through relay proton transfers with the help of auxiliary water (W2) yielding HPO42− and ADP, as a product. In the next step, a proton is shifted back from Glu1742 to generate inorganic phosphate (H2PO4−) via another relay proton transfer event. The overall activation barrier for the Glu1742 assisted ATP hydrolysis is found to be the most favourable pathway compared to other plausible pathways. We also unearthed that ATP hydrolysis in dynein follows a so-called associative-like pathway in its rate-limiting step. Our study ascertained the important indirect roles of the two amino acids (such as Arg2109, Asn1792) and Mg2+ ion in the ATP hydrolysis of dynein. Additionally, multiple sequence alignment of the different organisms of dynein motors has conveyed the evolutionary importance of the Glu1742, Asn1742, and Arg2109 residues, respectively. As similar mechanisms are also prevalent in other motors, and GTPase and ATPase enzymes, the present finding spells out the definitive requirement of a proton relay process through an extended water-chain as one of the key components in an enzymatic ATP hydrolysis reaction.


 Please wait while we load your content...
Please wait while we load your content...