Application of a tetrapyrimidyl cyclobutane synthesized in the organic solid state: a halogen-bonded supramolecular ladder†
Abstract
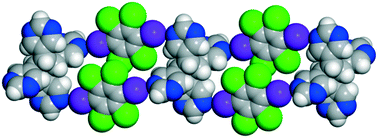
A halogen-bonded supramolecular ladder comprised of a novel pyrimidine-based cyclobutane photoproduct synthesized in the organic solid state via a [2 + 2] photoreaction is reported. The photoproduct rctt-tetrakis(5′-pyrimidyl)cyclobutane functions as rungs while the linear divergent halogen-bond donor 1,4-diiodoperchlorobenzene acts as the rails. Our report also confirms the structure and stereochemistry of the tetrapyrimidyl cyclobutane ring system.
- This article is part of the themed collection: Introducing the CrystEngComm Advisory Board and their research


 Please wait while we load your content...
Please wait while we load your content...