Metal–organic frameworks with solvent-free lanthanide coordination environments: synthesis from aqueous ethanol solutions†
Abstract
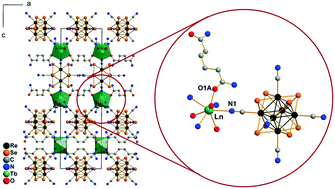
A series of 12 new metal–organic frameworks based on lanthanide cations (Ln = Nd, Sm–Dy), octahedral cluster anions [Re6S8(CN)6]4− or [Re6Se8(CN)6]4− and adipamide (adp) were synthesized under mild conditions in aqueous ethanol solutions. Compounds of the general composition Cs[Ln(adp)2{Re6Q8(CN)6}]·3H2O (Ln = Nd, Sm–Dy; Q = S or Se) crystallize in the hexagonal space group P6422. Despite the presence of solvate H2O molecules in structure cavities, the crystal structures revealed that the coordination environment of lanthanide cations in these 3D polymers did not contain H2O ligands. This rare feature is attributed to the combination of a bulk cluster anion and flexible adp molecules, which occupy the lanthanide coordination sphere to yield a stable lanthanide-based building block. This assumption was confirmed by synthesis of a second crystalline modification of the compound Cs[Nd(adp)2{Re6S8(CN)6}]·3H2O (orthorhombic space group Cccm). Luminescence studies evidence the presence of the typical red luminescence of hexarhenium cluster complexes and the absence of observable luminescence of lanthanide cations for all the compounds except the Nd derivatives. Magnetic data indicate very weak antiferromagnetic exchange interactions between paramagnetic Ln3+ centers.
- This article is part of the themed collection: Editor’s Collection: Rare Earth Materials


 Please wait while we load your content...
Please wait while we load your content...