Substituent effects of auxiliary ligands in mononuclear dibenzoylmethane DyIII/ErIII complexes: single-molecule magnetic behavior and luminescence properties†
Abstract
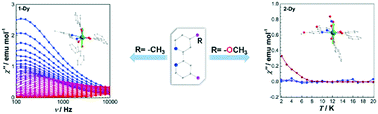
Four mononuclear rare earth complexes, [(dbm)3Ln(dmbipy)] (1-Ln, Ln = Dy, Er) and [(dbm)2Ln(dmobipy)(NO3)] (2-Ln, Ln = Dy, Er) (Hdbm = dibenzoylmethane, dmbipy = 4,4′-dimethyl-2,2′-bipyridine, dmobipy = 4,4′-dimethoxy-2,2′-bipyridine), have been synthesized by tuning the substituents at the 4,4′-positions of 2,2′-bipyridine. It is interesting to note that replacing the substituents of auxiliary ligands from –CH3 to –OCH3 results in a major difference in the molecular formula and geometries. Magnetic measurements show that slow relaxation of the magnetization appears clearly in the absence of a dc field for 1-Dy, while under an applied dc field for the others. Ab initio calculations reveal that the calculated effective gz value for 1-Dy is larger than that for 2-Dy, consistent with the experimental results. In addition, the luminescence properties of the four complexes have been studied, and 2-Dy, 1-Er and 2-Er can exhibit characteristic emissions of DyIII/ErIII ions.


 Please wait while we load your content...
Please wait while we load your content...