Structures and transistor properties of extended and unsymmetrical birhodanines†
Abstract
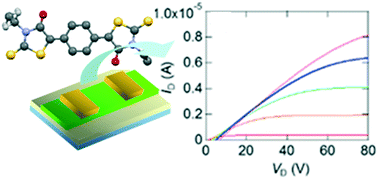
Birhodanines exhibit n-channel transistor properties with air stability. In this work, birhodanines with extended skeletons are investigated, in which a phenylene or quinoidal moiety is inserted into the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C part. We have also prepared N-phenyl and unsymmetrically N-substituted derivatives, including an unsubstituted N-H part. These compounds show n-channel transistor properties. In contrast to the herringbone structure of the parent compounds, the phenylene and phenyl compounds have stacking structures. The phenylene substitution decreases the acceptor ability, whereas the quinoidal substitution improves the acceptor ability and air stability of the transistors. The N-H derivative has a Z-form, suggesting the contribution of the enolic form, and the unsymmetrically N-substituted derivatives have double-layer structures with ordered alkyl chains.
C part. We have also prepared N-phenyl and unsymmetrically N-substituted derivatives, including an unsubstituted N-H part. These compounds show n-channel transistor properties. In contrast to the herringbone structure of the parent compounds, the phenylene and phenyl compounds have stacking structures. The phenylene substitution decreases the acceptor ability, whereas the quinoidal substitution improves the acceptor ability and air stability of the transistors. The N-H derivative has a Z-form, suggesting the contribution of the enolic form, and the unsymmetrically N-substituted derivatives have double-layer structures with ordered alkyl chains.


 Please wait while we load your content...
Please wait while we load your content...