Methylene spacer regulated variation in supramolecular assembly of zinc(ii) dicyanamide complexes with reduced Schiff base ligands: synthesis, structure and DFT study†
Abstract
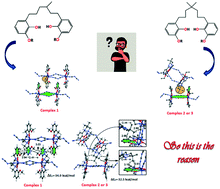
Three tetranuclear zinc dicyanamide complexes with three potential hexadentate reduced Schiff base ligands have been synthesized and characterized. In the solid state structure of complex 1, the aromatic ring of one complex is located in the U-cavity formed by two aromatic rings and the (Zn2)L2 (dicyanamide)2 macrocycle of the adjacent complex establishing π⋯π and C–H⋯π interactions. In sharp contrast, the presence of two methyl groups in addition to the longer methylene chain in 2 and 3 provokes a totally different packing, where the methyl groups are located in the U-cavity instead of the aromatic ring, establishing several C–H⋯π contacts. Thus, the presence of a methyl spacer and an additional methyl substituent in the alkyl chain of 2 and 3 is crucial since it has an enormous effect on the crystal packing. The DFT study is devoted to the analysis of the competition between C–H⋯π and π⋯π interactions observed in the solid state of complexes 1 and 2 and also to the characterization of the interactions using the NCI (non-covalent interaction) plot index computational tool. The molecular electrostatic potential (MEP) surface of complex 2 as a model of complexes 1–3 has also been calculated.


 Please wait while we load your content...
Please wait while we load your content...