Thermal expansion and dimensionality of a hydrogen bond network: a case study on dimorphic oxalic acid†
Abstract
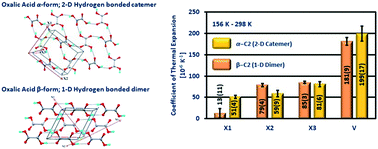
Anhydrous oxalic acid (C2) has two polymorphic forms – the orthorhombic α form (α-C2) and the monoclinic β form (β-C2). The structure of α-C2 is a two-dimensional hydrogen bonded sheet, sustained by a catemeric O–H⋯O hydrogen bond. The structure of β-C2 is a one-dimensional hydrogen bonded chain of C2, formed by the carboxylic acid dimers. Between 156 K–298 K, α-C2 exhibits a volumetric thermal expansion of 199(17) [10−6 K−1], while β-C2 exhibits a volumetric expansion of 181(9) [10−6 K−1]. Even though the crystal structures of the C2 polymorphs are sustained by hydrogen bonds of different dimensionalities, the volumetric thermal expansion of both forms are comparable. The structure–property study reveals that the dimensionality of hydrogen bonds in the structure influences the anisotropy of expansion in both the polymorphs. The volumetric thermal expansion of the polymorphs depends on the nature of the interactions and crystal packing.


 Please wait while we load your content...
Please wait while we load your content...