Polymorphism in natural alkamides from Aniba riparia (Nees) Mez (Lauraceae)†
Abstract
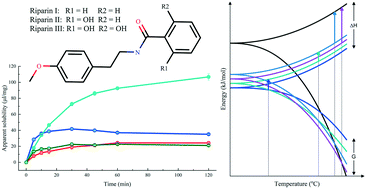
The polymorph selection during the discovery and development of new drugs is a crucial factor in ensuring the efficacy of a formulation. Thus, the physicochemical properties, including crystal structure, need to be considered in the decision tree that leads to a drug candidate. Riparins, natural alkamides isolated and later synthesized from Aniba riparia, have demonstrated potential therapeutic activities in preclinical studies, such as antidepressant, anxiolytic, and anti-inflammatory effects. Therefore, detailed physicochemical characterization is required as a starting point in the development of a new formulation. This contribution aims to study the polymorphism of three riparins, namely I, II, and III. The elucidation of the crystal structures of several polymorphs combined with thermal analysis studies allowed the determination of the thermodynamic relationships of the polymorphic pairs. The solubility of the three riparins was determined through the measurement of the corresponding dissolution profiles.


 Please wait while we load your content...
Please wait while we load your content...