Cu(i) Iodide coordination polymers with aromatic thioamides†
Abstract
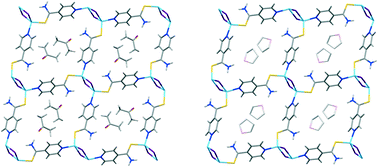
The reaction of 4-pyridinethiobenzamide (PyTBA) with CuI and KI aqueous solution in acetone or THF as a solvent under slow diffusion gives rise to the formation of single crystals of two coordination polymers of formula [CuIPyTBA]n·nS (S = acetone (1); 1/3THF (2)). X-ray diffraction analysis of compounds 1 and 2 shows that they consist of a 2D layered network formed of Cu2I2 units linked both by pyridinic N and S atoms from two PyTBA ligands. The metal–organic network creates voids that are occupied by the solvent molecules. The similar direct reaction carried out at room temperature between a mixture of CuI and PyTBA in acetone leads to the isolation of a microcrystalline red solid which is a mixture of [CuIPyTBA]n·n acetone (1) and [CuIPyTBA]n (3). The thermal treatment of this mixture allows 3 to be isolated in its pure form. The structure from the X-ray diffraction data of 3 reveals that it consists of a 3D interpenetrated non-porous network of formula [CuIPyTBA]n (3). The exposure of 3 to acetone leads to a mixture of 1 and 3, while the heating of this mixture results in a complete transformation into 3. This desolvated–solvated transformation observed in 3 has been analysed and rationalized based on the X-ray structures of the coordination polymers.


 Please wait while we load your content...
Please wait while we load your content...