Synthesis and structures of polyiodide radical cation salts of donors combining tetrathiafulvalene with multiple thiophene or oligo-thiophene substituents†
Abstract
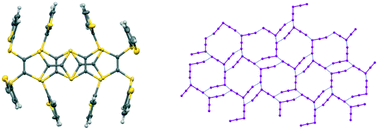
A series of TTF and EDT-TTF derived donors bearing thiophene, bithiophene and terthiophene side chains is described, from which a group of six radical cation salts with polyiodide ions were formed and structurally characterised. Typically, they contain complex networks of pentaiodide or triiodide anions or both, in some cases including further iodine, with networks of eight, ten, twelve or sixteen iodine atoms. Donor monocations form face-to-face pairs, but tetrakis-substituted donors are slipped along their main axis so the donor side chains can wrap around each other.


 Please wait while we load your content...
Please wait while we load your content...