Rational design, supramolecular synthesis and solid state characterization of two bicomponent solid forms of mebendazole†
Abstract
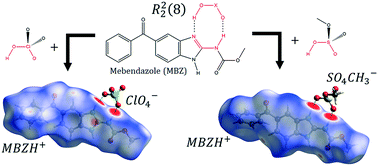
Two novel bicomponent solid forms of mebendazole (MBZ) were developed as a means to modulate its psychochemical and pharmaceutical properties. Supramolecular synthesis of MBZ A or C with perchloric or methyl sulfuric acids yields two salts of 1 : 1 stoichiometry, which were analyzed through single crystal X-ray diffraction. MBZ perchlorate crystallizes in the P21/n space group, while MBZ methyl sulfate crystallizes in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The API and its counterions are interconnected in both crystal packings by an R22(8) supramolecular motif. The 3-dimensional crystal structure is also stabilized by other strong intermolecular hydrogen bonds as well as non-classical interactions. FT-IR spectra are consistent with the inclusion of the anions in the crystal structure. MBZ perchlorate is stable up to 210 °C when it undergoes endothermic loss of the ester moiety. MBZ methyl sulfate is stable up to 160 °C when endothermic elimination of the methylcarbamate moiety occurs. The solubility behavior of MBZ perchlorate, studied by UV-visible spectroscopy, suggests an improvement by a factor of seven with respect to MBZ A, in the apparent solubility of MBZ in 0.1 mol L−1 HCl. Further experiments are required to evaluate both the stability of the solids and the maximum solubility of the API.
space group. The API and its counterions are interconnected in both crystal packings by an R22(8) supramolecular motif. The 3-dimensional crystal structure is also stabilized by other strong intermolecular hydrogen bonds as well as non-classical interactions. FT-IR spectra are consistent with the inclusion of the anions in the crystal structure. MBZ perchlorate is stable up to 210 °C when it undergoes endothermic loss of the ester moiety. MBZ methyl sulfate is stable up to 160 °C when endothermic elimination of the methylcarbamate moiety occurs. The solubility behavior of MBZ perchlorate, studied by UV-visible spectroscopy, suggests an improvement by a factor of seven with respect to MBZ A, in the apparent solubility of MBZ in 0.1 mol L−1 HCl. Further experiments are required to evaluate both the stability of the solids and the maximum solubility of the API.


 Please wait while we load your content...
Please wait while we load your content...