New nitrogen-rich heterocyclic compounds to build 3D energetic metal complexes†
Abstract
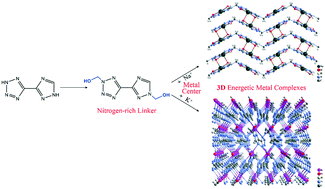
2-Hydroxymethyl-5-(2-hydroxymethyl-tetrazole-5-yl)-triazole (2) was designed and obtained in a good yield by the reaction of (tetrazol-5-yl)-triazole (1) with formaldehyde in the presence of 20% H2SO4. Three dimensional (3D) energetic metal complexes, sodium and potassium salts of 1 and 2 (3 and 4 for 1 and 5 and 6 for 2, respectively), were synthesized and thoroughly characterized. The crystal structures of 2–6 were confirmed by using single crystal X-ray diffraction. Because of the presence of hydroxymethyl in 2, 5 and 6 formed fascinating 3D supramolecular network architectures, which show more remarkable closer packing and higher density than 3 and 4. All the compounds are insensitive to impact and friction, and exhibit high thermal stabilities. The Hirshfeld surface calculation reveals that 2 has strong intermolecular interactions. In these cases, the method of introducing hydroxymethyl into energetic nitrogen-rich heterocyclic compounds results in superior structure and performance.


 Please wait while we load your content...
Please wait while we load your content...